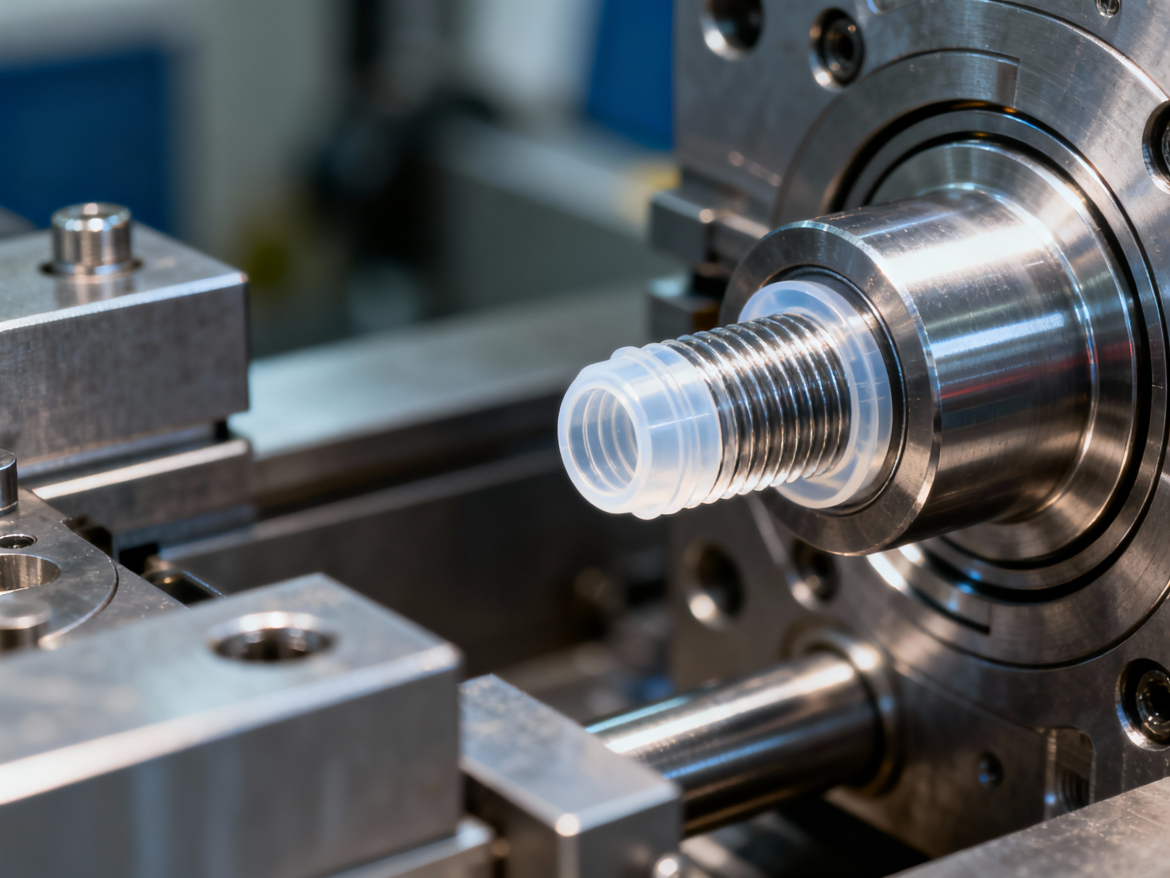
For medical components requiring rigorous thread integrity, such as dosing caps, Luer connectors, and surgical fluid transfer devices, forced ejection often proves unsuitable. While stripping molds offer lower initial tooling costs, they introduce significant risks of particulate generation and dimensional deformation, which are unacceptable in sterile environments. The superior alternative is unscrewing plastic injection molding China technology, which utilizes rotating cores to safely unthread parts during the mold open cycle, ensuring zero-draft precision. Partnering with a capability-driven plastic injection molding in China manufacturer allows engineering teams to access these complex tooling technologies and cleanroom standards at a scalable, cost-effective level. This approach ensures strict adherence to ISO requirements without compromising on thread quality or functional sealing performance.
Optimizing Thread Integrity via Advanced Unscrewing Mechanisms
Manufacturing threaded components requires selecting between forced stripping and rotational unscrewing. This decision impacts cycle time, material compatibility, and dimensional stability, particularly for high-volume medical applications requiring leak-proof performance and particulate-free surfaces.
Limitations of Forced Stripping Protocols
Forced ejection, or “bump-off,” relies on the polymer’s elasticity to expand and slip over steel threads during ejection. While effective for simple bottle caps using low-modulus materials like Polypropylene (PP) or Polyethylene (PE), this method fails when applied to rigid engineering resins required for medical devices. High-modulus materials, such as Polycarbonate (PC) or glass-filled PEEK, typically exhibit elongation at break values below 50%, leading to thread shearing or stress whitening during stripping. Data indicates that forced ejection is generally viable only if the thread depth is shallow and the material allows for approximately 5% strain without permanent deformation. Beyond these limits, the risk of ovalization increases significantly, compromising the circularity required for hermetic seals in Luer fittings. Furthermore, the friction generated during stripping can create microscopic plastic dust, violating particulate standards in cleanroom molding environments.
Engineering Zero-Draft Geometries
To overcome stripping limitations, unscrewing plastic injection molding China specialists employ automatic unscrewing molds. These tools utilize a rack-and-pinion system or hydraulic motors to rotate threaded cores in synchronization with the mold opening sequence. Modern systems increasingly integrate electric servo motors, which provide precise torque control and positional accuracy down to 0.01mm. This technology allows for the production of parts with deep, multi-start threads and zero-draft geometry, which is physically impossible to eject forcibly. For example, a servo-driven unscrewing mold can produce a complex dosing dial with internal threads while maintaining a cycle time comparable to standard molds by overlapping the unscrewing phase with the cooling cycle. This mechanical synchronization ensures that high-precision threaded features retain their exact CAD-specified geometry without the mechanical stress associated with stripping.
Livepoint Tooling: Precision Engineering for Complex Threaded Molds
Livepoint Tooling integrates twenty-three years of expertise with advanced manufacturing capabilities. Their vertical integration from mold fabrication to mass production supports global medical OEMs in delivering compliant, high-precision threaded devices through resilient supply chain strategies.
Manufacturing Infrastructure and Quality Compliance
Livepoint operates a robust manufacturing ecosystem designed for high-stakes medical applications. Their facility manages plastic injection molding service China operations under strict quality management systems, holding ISO 9001 and IATF 16949 certifications, while actively aligning processes with ISO 13485 protocols for medical device compliance. This infrastructure includes controlled cleanroom environments capable of supporting Class I, II, and III medical device production, effectively minimizing bio-burden and particulate contamination. For complex threaded parts, Livepoint utilizes advanced metrology equipment, including CMM and non-contact optical inspection, to verify thread pitch and diameter within micron-level tolerances. This rigorous validation ensures that every batch meets the functional requirements of FDA and EU MDR regulations, providing full lot traceability from the raw resin to the finished component.
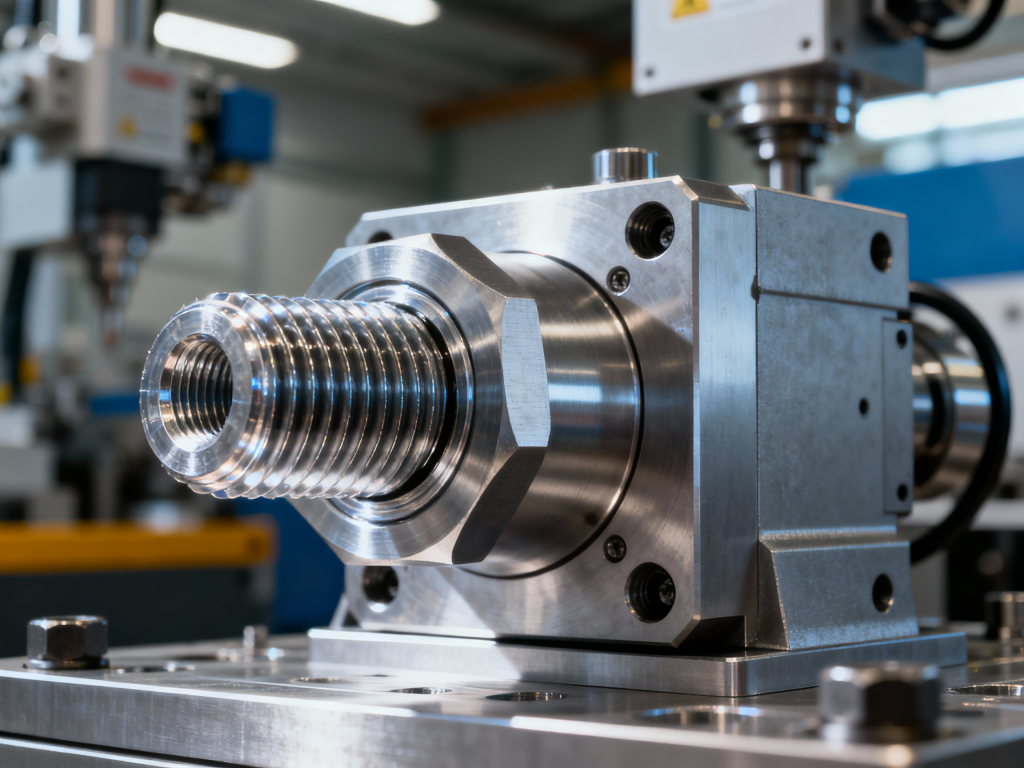
Scalable Tooling from Prototype to Production
Livepoint distinguishes itself among custom plastic injection molding services suppliers by offering scalable tooling solutions tailored to project lifecycles. For early-stage clinical trials, they fabricate rapid aluminum bridge tooling, enabling the production of representative threaded parts in as little as two weeks. As demand scales, the transition to Class 101 hardened steel molds ensures consistent output for millions of cycles. Their in-house tooling shop features high-precision CNC machining and EDM capabilities, essential for crafting the intricate gears and collapsing cores used in unscrewing molds. This capability allows for the dual-sourcing of molds and parts, mitigating global logistics risks. By leveraging precision plastic injection molding China expertise, Livepoint delivers cost-efficiency without sacrificing the mechanical complexity required for sophisticated medical assemblies.